Safety and efficacy of allogenic adipose tissue-derived mesenchymal stromal cells in patients with epidermolysis bullosa: clinical trial phase I/II
MSC-EB is a phase I/II clinical trial aiming to evaluate the safety and efficacy of the mesenchymal stromal cell-based medicinal product in patients with the rare congenital (genetic) disease epidermolysis bullosa (i.e., butterfly wing disease). In the first phase of the study, the medicinal product will be administered to adult patients, and in the second phase to children with this disease.
The medicinal product was developed and manufactured by the CZECRIN GMP unit ACIU MU. Its basis consists of specialized cells isolated from adipose tissue of healthy donors. Every person has these cells in their body, where they repair damaged tissues and influence the functions of the immune system. These cells can naturally suppress the excessive inflammatory reaction in difficult-to-heal skin wounds. Administration of mesenchymal stromal cells could accelerate chronic wound healing in patients with butterfly wing disease.
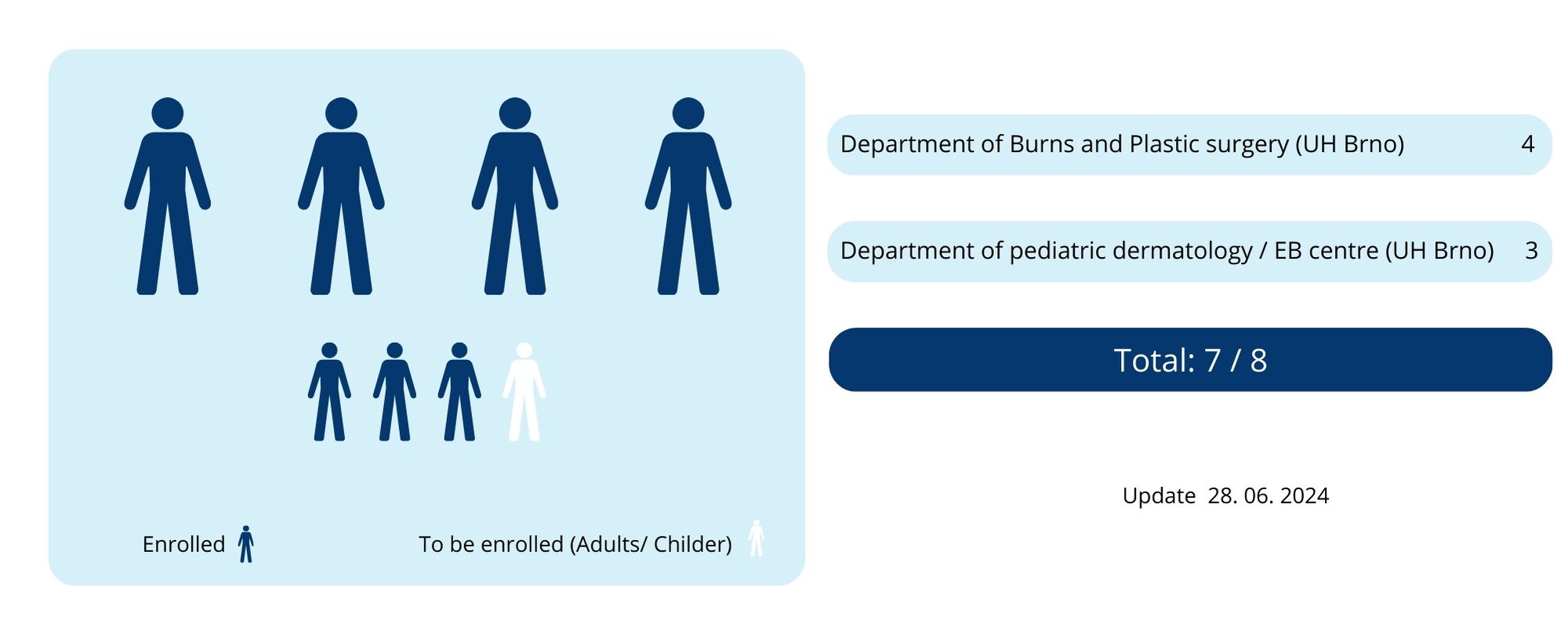
Masaryk University is the sponsor of the trial. Patients participating in the study are in the medical care of the principal investigator, Jitka Vokurková, MD, PhD and her colleagues from the Department of Burns and Plastic Surgery, and Hana Bučková, MD, PhD from the Department of pediatric dermatology/EB Center of the Brno University Children Hospital.
Patient organization DEBRA ČR helps and supports the trial project from its beginning.
The recruitment of patients has started in October 2022.