Combined antitumor therapy with ex vivo manipulated dendritic cells producing interleukin-12 in children, adolescents and young adults with progressive, recurrent or primarily metastatic high-risk tumors
KDO_DC1311 is a phase I/II clinical trial aiming to evaluate the safety and efficacy of the dendritic cell-based medicinal product in children, adolescent and young adult cancer patients.
The medicinal product was developed and manufactured by the CZECRIN GMP unit ACIU MU. The basis of the medicinal product is the patient’s immune cells and a sample of the patient’s tumor tissue. In the laboratory, a specific type of collected immune cells is matured and learned to recognize the tumor cells. After administering these trained immune cells, the patient’s antitumor immunity should be supported.
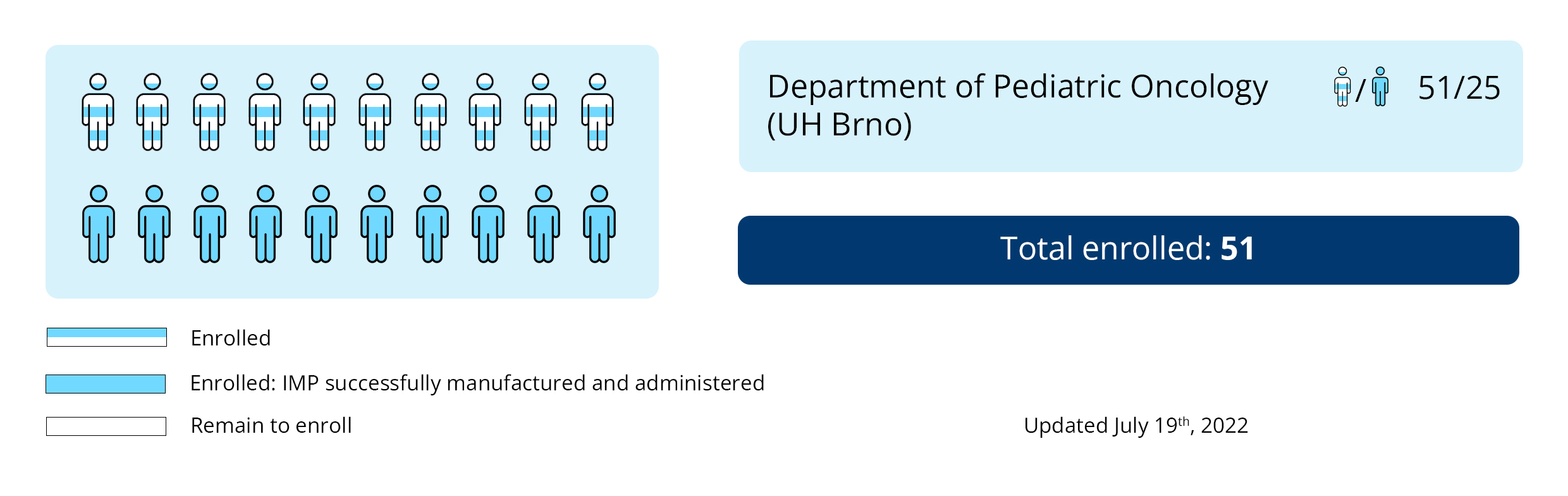
Masaryk University is the sponsor of the trial. The patients participating in the study are in the medical care of the principal investigator Peter Múdry, MD, PhD and his colleagues from the Department of Pediatric Oncology at the Brno University Hospital. Patients undergo all available anti-cancer treatments in parallel with the tested medicinal product.
Recruitment to the KDO_DC1311 trial has already been completed. Follow-up visits of enrolled patients continue at the Department of Pediatric Oncology, where the investigators monitor the health status of the patients and their disease course.