M.A.R.S. is a randomized, double-blind, placebo-controlled, multicenter study of the efficacy and safety of highly diluted atropine eye drops in slowing the progression of myopia in children, a phase IIIb, three parallel-arm study designed to increase clinical knowledge of the effect of topical application of highly diluted atropine on the growth of axial length in myopic eyes and supplementary experimental knowledge about the effectiveness and safety of this application.
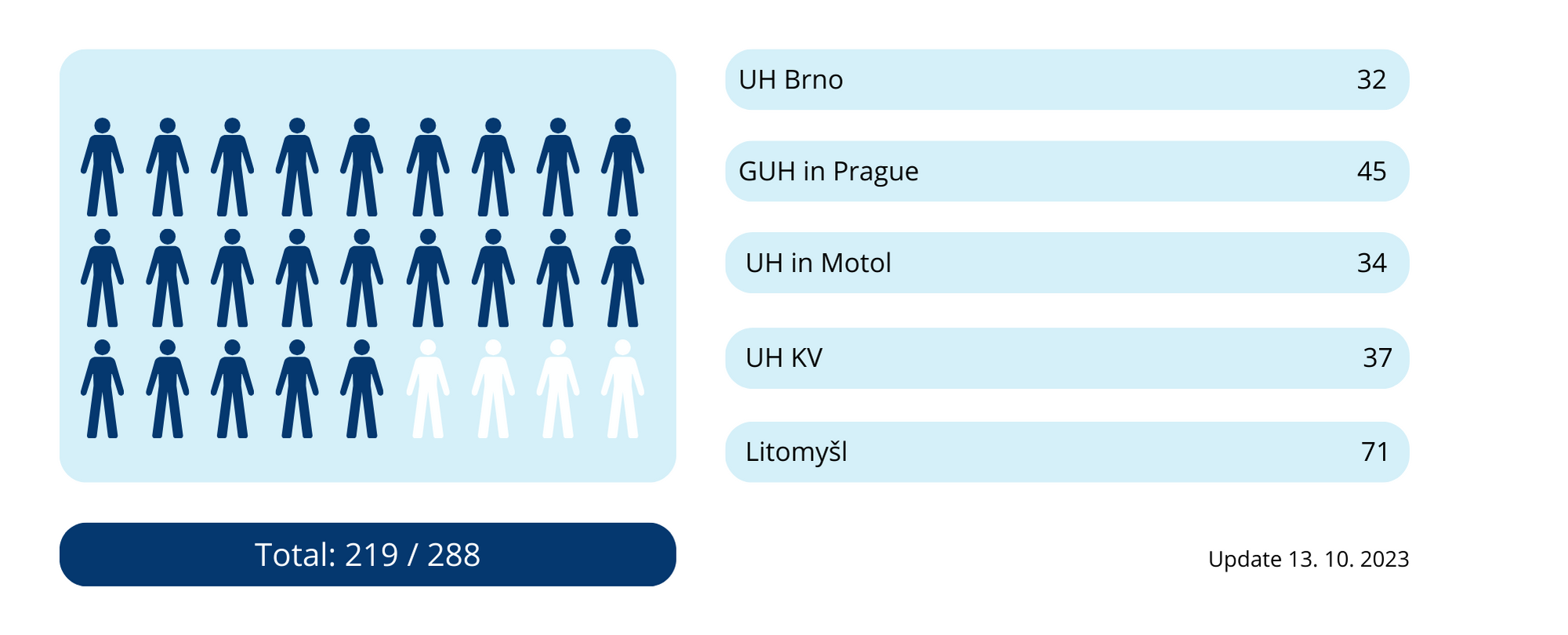
The study was initiated by the University Hospital Brno in cooperation with Masaryk University. This is a pediatric clinical trial, which will include in the total of 288 patients in 5 clinical trial centers in the Czech Republic.
The study was initiated in the Czech Republic on June 29, 2022 and the recruitment will continue until the target number of patients is enrolled.
As of October 13, 2023, 219 patients were included in the study.