Randomised Placebo-Controlled Trial of Early Targeted Treatment of Patent Ductus Arteriosus with Paracetamol in Extremely Low Birth Weight Infants
The objective of the study is to determine whether, among extremely low birth weight (ELBW – birth weight less than 1000g) infants, early targeted treatment with Paracetamol for patent ductus arteriosus (PDA), based on specific criteria and commencing between six to twelve hours of life, results in significant reduction of a combined primary outcome of periventricular and intraventricular haemorrhage (PIVH), necrotising enterocolitis (NEC) and death before discharge. The Trial design is a Phase 3 randomised, parallel-group, placebo-controlled trial.
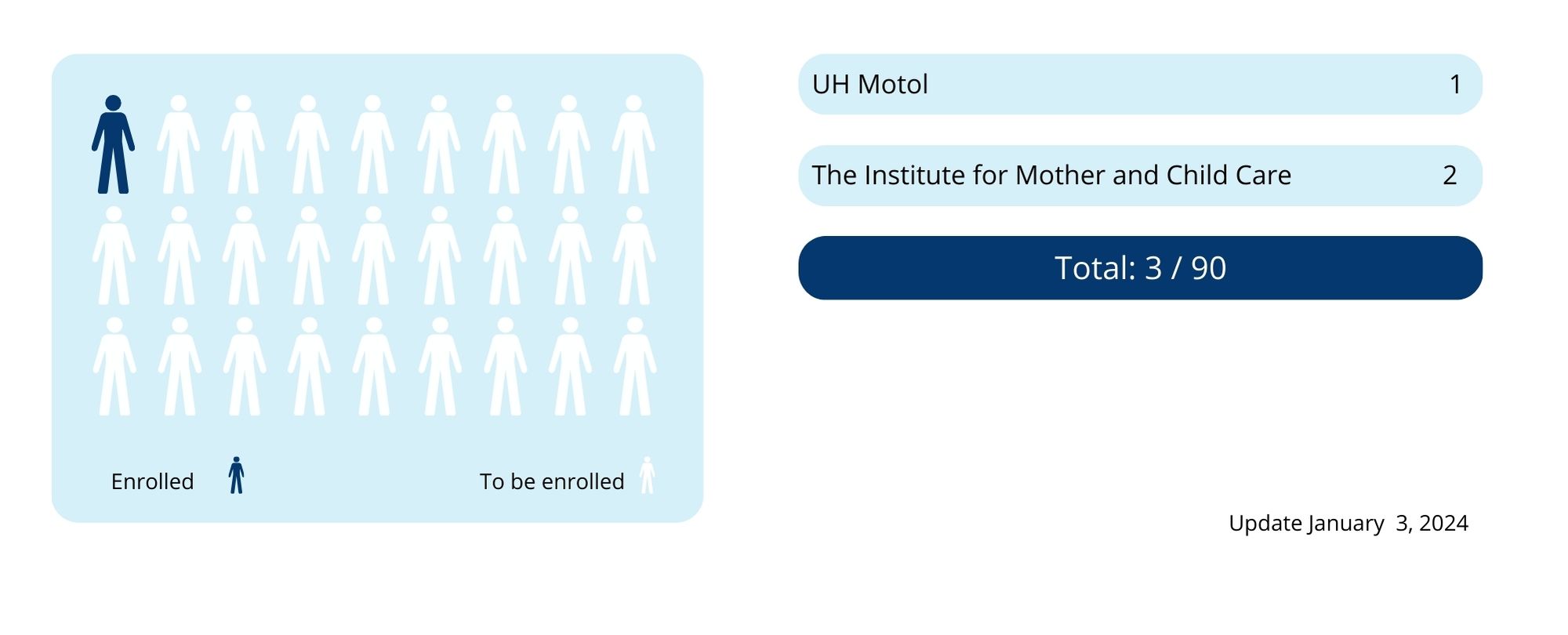
The study was commissioned by University College Dublin, Ireland, and led by Principal Investigator Professor Jan Miletín. In the Czech Republic, the clinical trial is being conducted at two centres in Prague – FN Motol and the Institute for Mother and Child Care in Podolí.
Both centres were initiated in September 2023. It is planned to include 90 patients, 45 in each centre. Patient recruitment will take approximately one year.
Eudra-CT: 2020-004245-37
The recruitment of patients has started in October 2022.