SEOTP is a Phase IV clinical trial to improve and innovate existing treatment approaches for psychosis based on genotype and endophenotype, including pharmacokinetic analysis, to minimise the adverse effects of olanzapine treatment and to provide further information on the efficacy of this treatment.
The clinical trial is conducted by Masaryk University. The patients participating in the trial are under the care of the principal investigators, Prof. Libor Ustohal, M.D., Ph.D., from the Psychiatric Clinic of the University Hospital Brno, and Petr Šilhán, M.D., Ph.D., from the Psychiatric Department of the University Hospital Ostrava, and their colleagues.
Patient recruitment
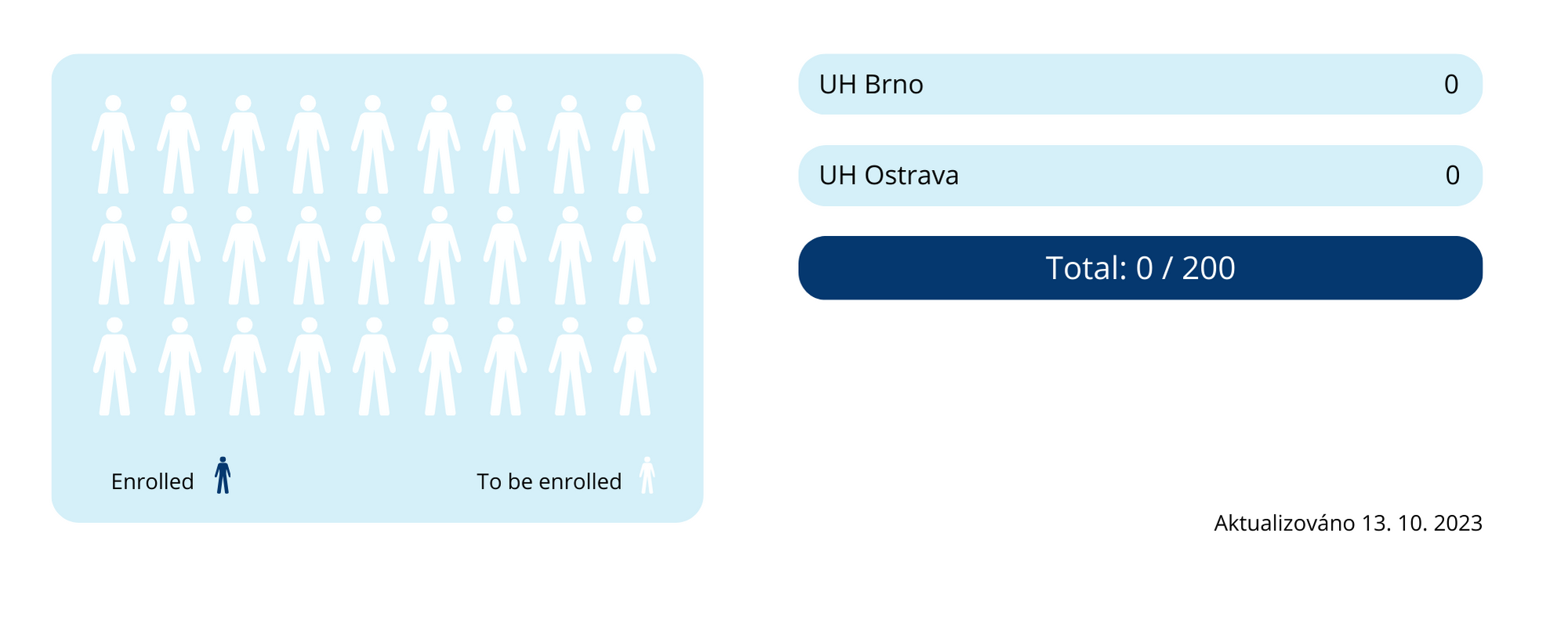
Clinical trials have been initiated in all participating centres but have not yet started. Of the total number of patients planned to be enrolled (200), 0 patients are currently enrolled.
Contact
SEOTP National Coordinator
Assoc. PharmDr. Jan Juřica, Ph.D.
Project Manager
Ing Karolína Grodová | karolina.grodova@med.muni.cz
RECRUITMENT OF PATIENTS