
Effect of dexamethasone in patients with ARDS and COVID-19 – prospective, multi-centre, open-label, parallel-group, randomized controlled trial (REMED trial). COVID-19 is still a significant challenge for medical research, exploring new drugs or possible optimizing the use of existing drugs. One way to use an existing medicine is to use the corticosteroid dexamethasone. REMED clinical trial aims to test the hypothesis that administration of dexamethasone 20 mg daily is more effective in the context of COVID-19 than a daily dose of dexamethasone 6 mg in adult patients with moderate or severe ARDS. This multicenter study initiator is the University Hospital Brno – Department of Anaesthesiology, Resuscitation and Intensive Care Medicine, which in cooperation with LRI CZECRIN carries out the study in 10 centres of the Czech Republic with 300 patients.
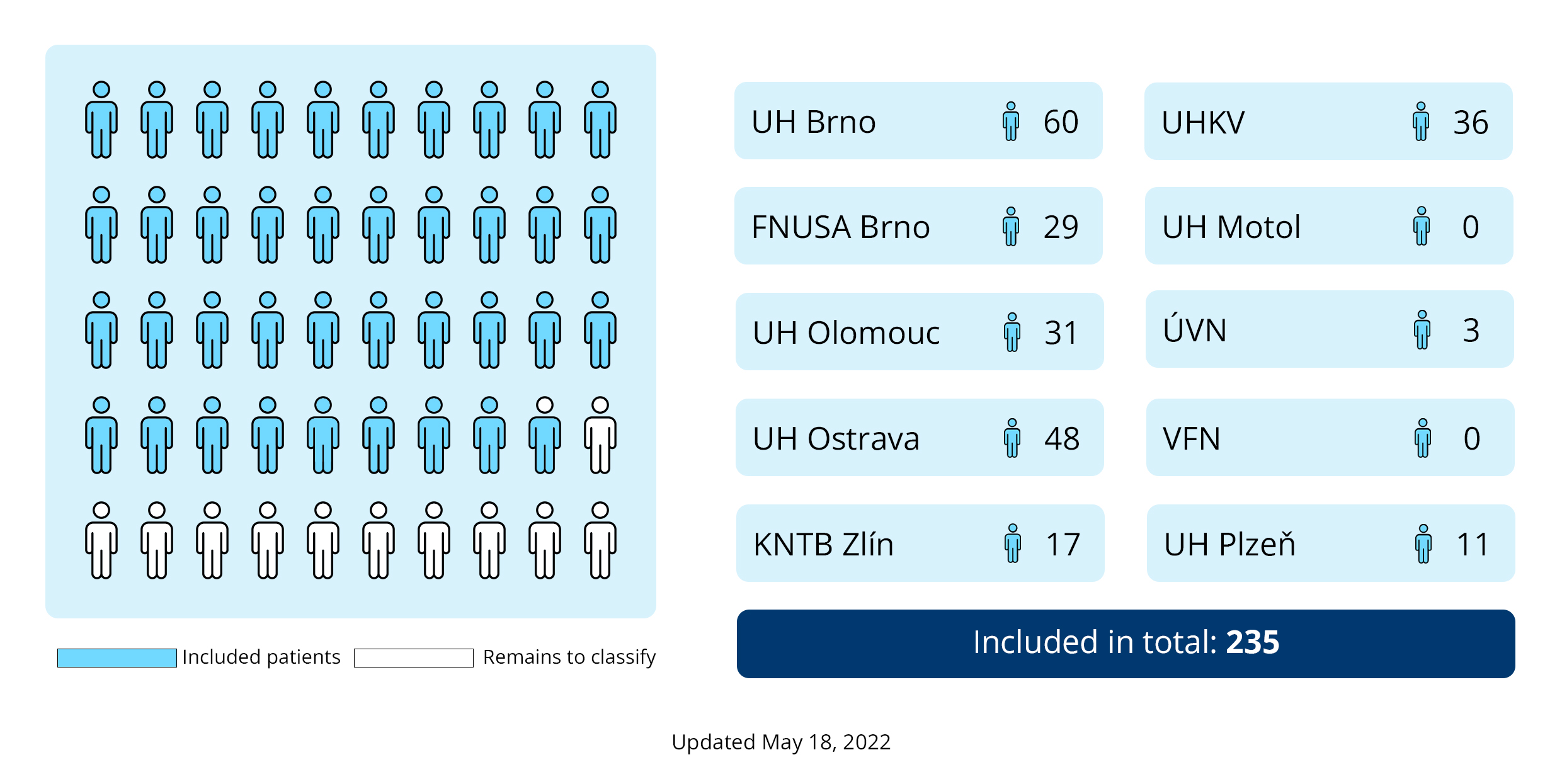
Recruitment to the REMED clinical trial was terminated on March 9, 2022 due to a receding pandemic. A total of 235 patients out of 300 were enrolled in the study. The infographic below shows the percentage of recruitment and the involvement of collaborating workplaces.
REMED is part of preplanned prospective meta-analysis of randomized trials comparing higher vs. standard doses of dexamethasone in patients with Covid-19 and Hypoxia – PMA protocol is registered here.
REMED Data and Safety Monitoring Board
Informed Concent Form (short version)