Prospective phase II clinical trial evaluating the efficacy and safety of tyrosine kinase inhibitor discontinuation after prior two-step dose reduction in patients with chronic myeloid leukemia in deep molecular remission
The HALF clinical trial is designed as a prospective, multicentre, non-randomized, open-label phase II study to investigate the efficacy and safety of tyrosine kinase inhibitor discontinuation after prior two-step dose reduction in patients with chronic myeloid leukemia in deep remission. The HALF clinical trial could contribute to the formulation of a new disease management strategy for patients achieving sustained remission with potentially more comprehensive benefits not only for the patients themselves but also for the healthcare system.
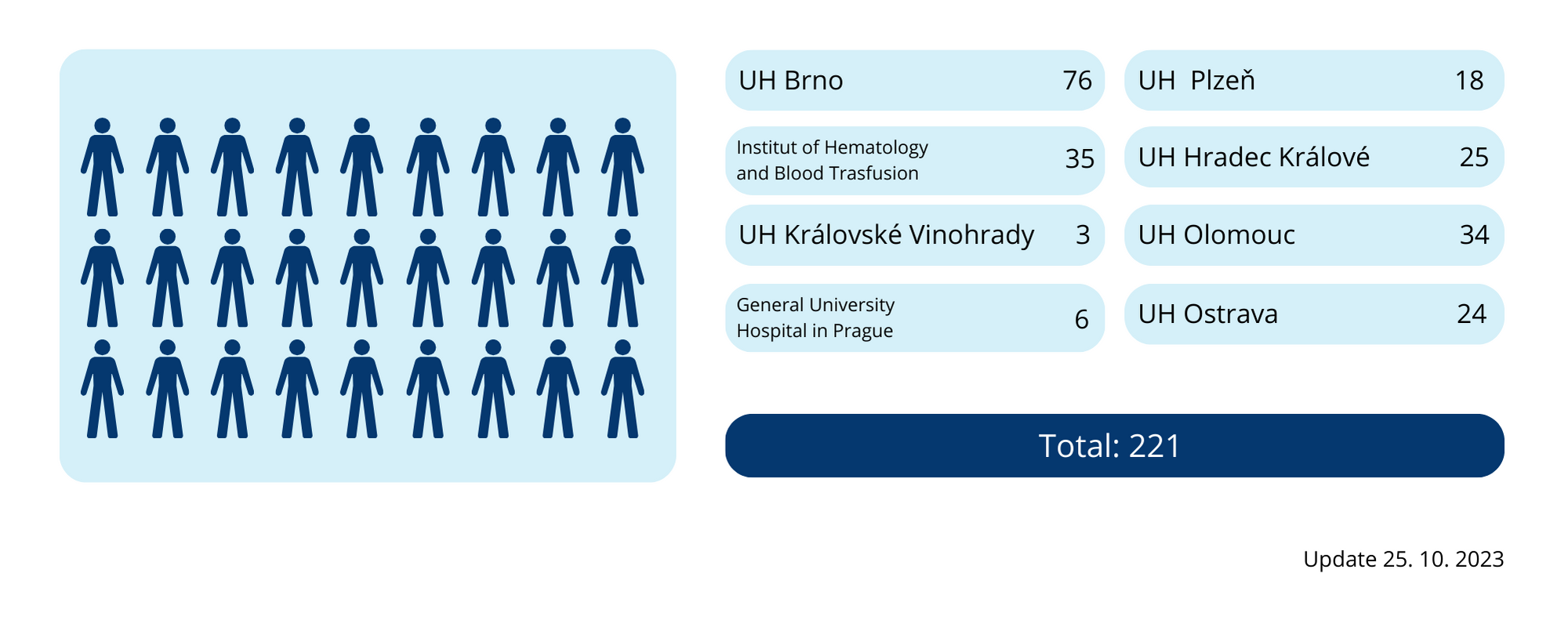
The planned number of subjects for the HALF trial was 150 subjects. This number was reached in January 2022. Recruitment was completed by June 30, 2023, with a total of 221 patients enrolled. The infographic below shows the collaborating sites’ involvement in terms of the number of subjects enrolled.