CLINICAL TRIAL MSC-EB
Safety and efficacy of allogeneic adipose-derived mesenchymal stromal cells in patients with epidermolysis bullosa: a phase I/II clinical trial
MSC-EB is a phase I/II clinical trial to evaluate the safety and efficacy of a mesenchymal stromal cell-based drug in patients with the rare congenital (genetic) disease epidermolysis bullosa (butterfly wing disease). In the first phase of the study, the drug will be administered to adult patients and in the second phase to children with this disease.
The medicinal product was developed and is manufactured by the CZECRIN GMP unit of ACIU MU. Its base consists of specialized cells isolated from adipose tissue of healthy donors. These cells are present in every person’s body, where they serve to repair damaged tissues and influence the functions of the immune system – they are able to naturally suppress the exaggerated inflammatory reaction that occurs even in difficult-to-treat skin wounds. Administration of mesenchymal stromal cells could accelerate the healing of chronic wounds in patients with butterfly wing disease.
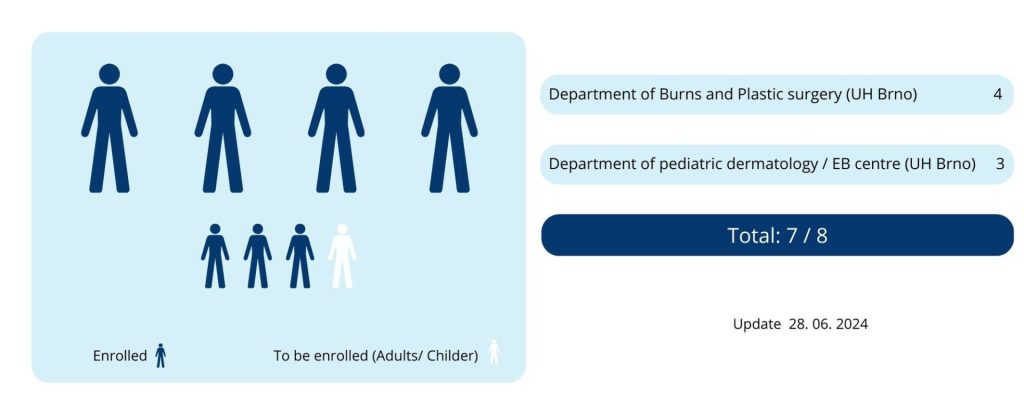
Patients participating in the study are under the care of the principal investigator, MUDr. Jitka Vokurková, Ph.D. and her colleagues from the Department of Burns and Plastic Surgery and MUDr. Hana Bučková, Ph.D. and her colleagues from the Children’s Skin Department/EB Centre of the University Hospital Brno.
The patient organisation DEBRA ČR, z. ú. has also been involved in the development of the product and the study from the beginning.
RECRUITMENT OF PATIENTS
Patient recruitment for the study began in October 2022.