CZECH
Clinical
Research
Infrastructure
Network
“…towards patient oriented medicine”
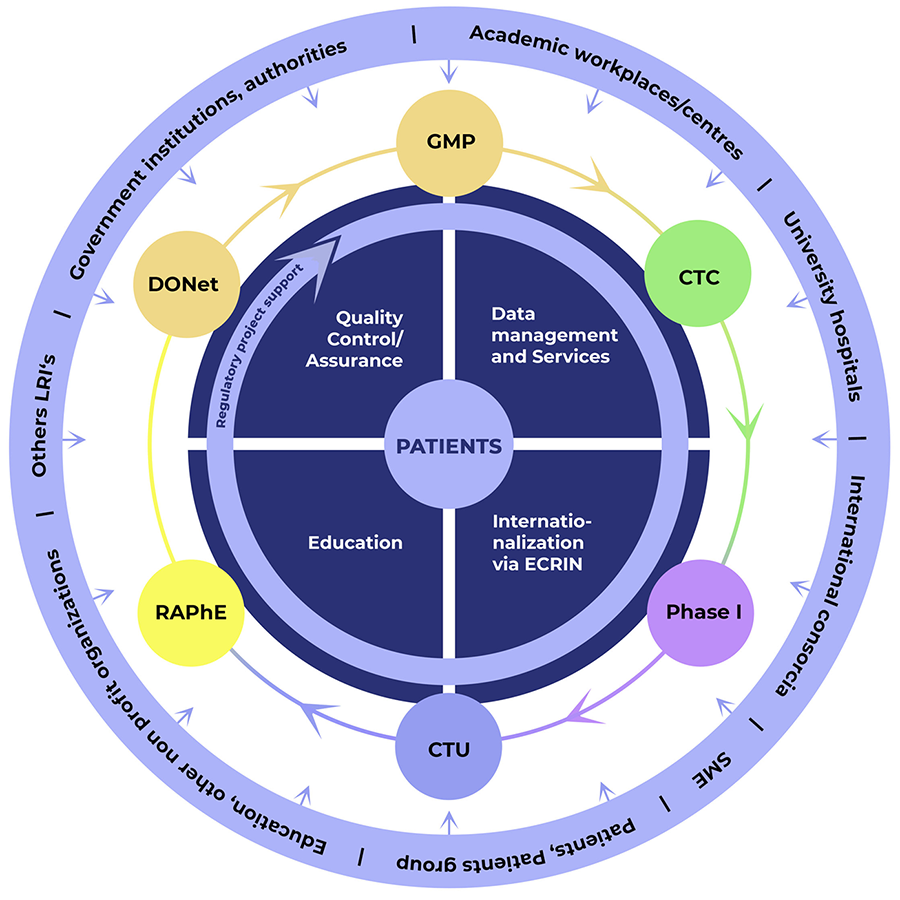
What is CZECRIN
CZECRIN is a key infrastructure supporting the implementation of academic clinical research in the Czech Republic. As the Czech node of the European Network of Clinical Research Infrastructures ECRIN-ERIC, it contributes substantially to the involvement of academic institutions in international clinical research projects. It has a unique network including most of the major clinical sites with clinical research orientation and provides knowledge, development, production and implementation capacities in the field of research and development of pharmaceuticals and medical devices. It sets the quality of processes and data with respect to the application of FAIR principles. CZECRIN is also a centre for cultivation and education in the field of clinical trials.
OUR EXPERTISE IN THE LIFE CYCLE
Regulation and Pharmacoeconomics (RAPhe)
Domain Oriented Networks (DONet)
Clinical Trials Team (CTC)
Clinical Trials Network (CTU)
European Clinical Research Implementation Network (ECRIN-ERIC)
Clean rooms for the production of ATMP for clinical research (GMP ATMP)
Clean rooms for the production of ATMP for clinical research (GMP ATMP)
Phase I units
Phase I units
Quality Assurance (QA)
Education
We offer our expertise and comprehensive services for the implementation of clinical research in the Czech Republic. We are happy to help you in the implementation of your project.
CZECRIN is the key infrastructure supporting the implementation of academic clinical research
12
hospital collaboration
92
clinical trials supported by CZECRIN
26
H2020 projects
68
participation in international projects
1600+
patients enrolled in the clinical trials
155
published articles
1500+
participants in seminars and conferences
4
innovative products in in-house development
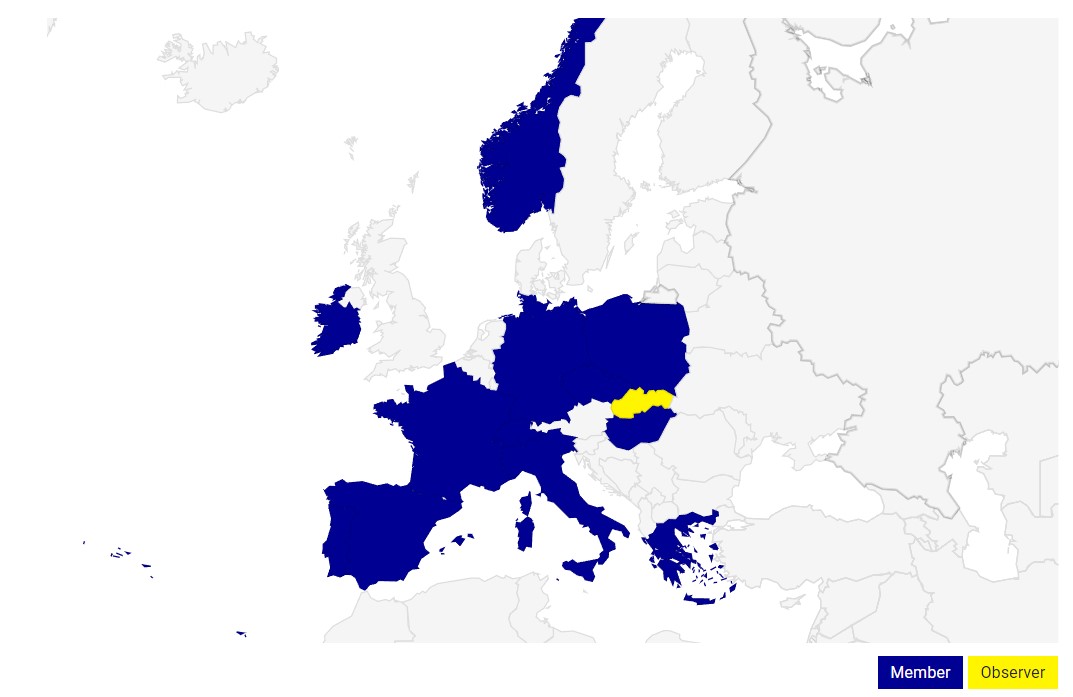
CZECRIN – NATIONAL NODE ECRIN-ERIC
CZECRIN represents the Czech national node of the pan-European research infrastructure ECRIN-ERIC (European Clinical Research Infrastructure Network), which enables the interconnection of clinically oriented research capacities in the Czech Republic with the research and application sphere in Europe and worldwide. The Czech Republic through CZECRIN is a full member of ECRIN-ERIC and part of ESFRI Roadmap. CZECRIN cooperates closely with other EU infrastructures such as BBMRI or EATRIS-ERIC at both national and international level and connects clinical research capacities of the Czech Republic associated within national networks. CZECRIN thus supports and enables the implementation of important transnational non-commercial clinical trials and active participation in pan-European projects supported by FP7, Horizon 2020 or IMI2.
CZECRIN NEWS
A regular flow of information not only on clinical trials
No Results Found
The page you requested could not be found. Try refining your search, or use the navigation above to locate the post.
Career
Do you wish to become a part of our CZECRIN multinational structure? Or even part of ECRIN? Find out what we can offer you right now.

