expertise and services
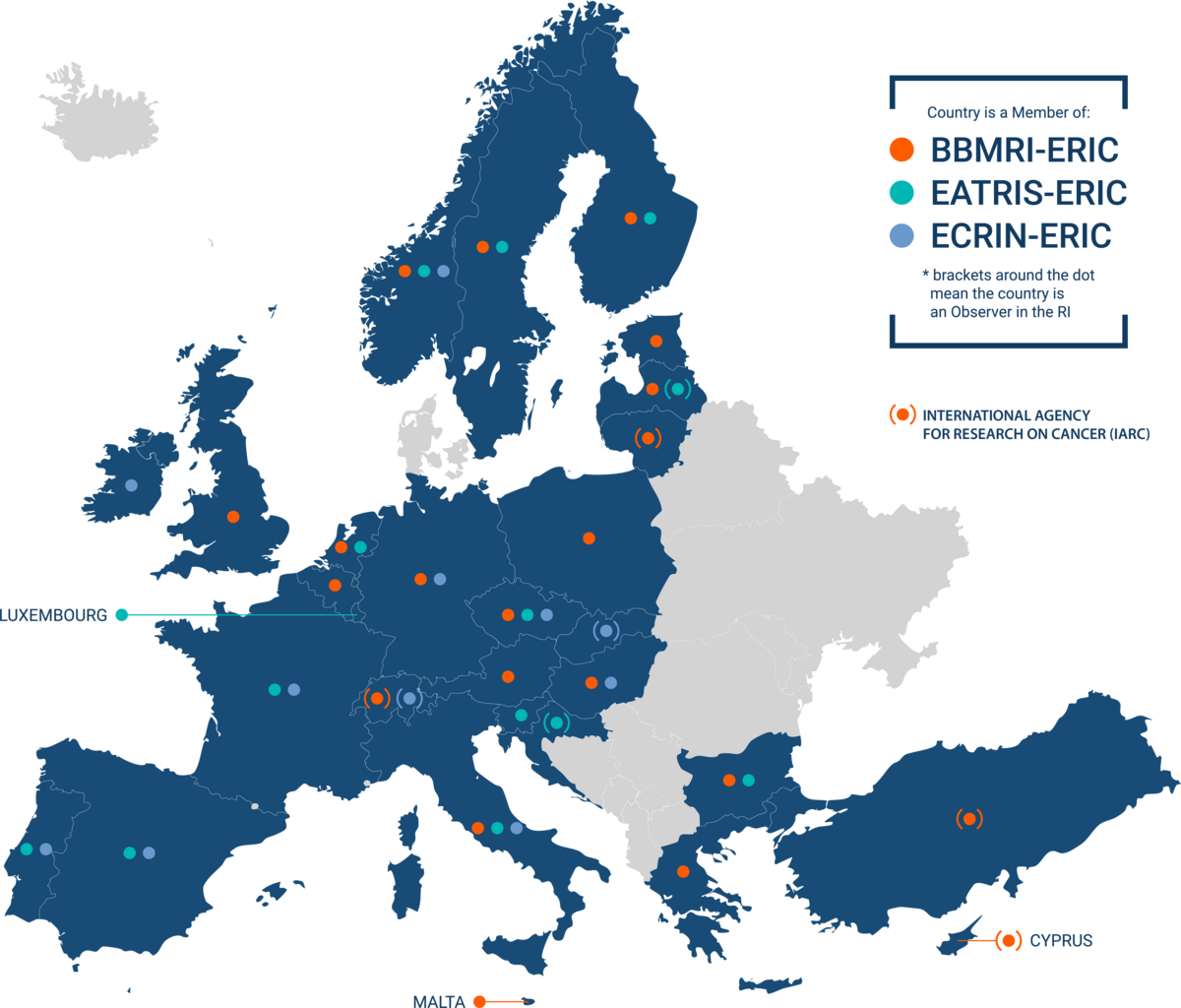
ECRIN-ERIC ATMP NODE
European methodological and coordination hub for non-commercial clinical trials of advanced therapies.
ECRIN-ERIC ATMP Node: clinical trials and European collaboration
ECRIN-ERIC ATMP Node is a European coordination and methodology centre focused on non-commercial clinical trials of advanced therapies (ATMPs). It promotes the link between scientific innovation, regulatory expertise and ensuring access to advanced treatments for patients within a single European environment.
The ATMP Node aims to strengthen Europe’s capacity to conduct high quality clinical trials through shared methodologies, harmonised regulatory procedures and decentralised research infrastructure.