GMP Core Units
About GMP Units
All products at all stages of the life cycle of a drug must be manufactured according to very strict guidelines and are rigorously controlled for human administration. This set of measures is known as Good Manufacturing Practice (GMP) and is a system of consumer protection in pharmaceutical manufacturing that minimises the risk of substandard or unsuitable medicines reaching the patient. This also applies to products used in clinical trials.
GMP UNITS
ACIU MUNI
CTEF-cGMP FNUSA-ICRC
GMP UNITS
ACIU MUNI
CTEF-cGMP FNUSA-ICRC

Flyer
Comprehensive summary of information on GMP units for download
Under Construction

GMP Projects
Complete database of projects and studies that have benefited from CZECRIN expertise
Under Construction
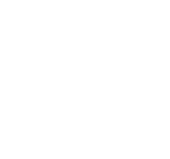
I am interested in co-operation
We offer our expertise to assist in the implementation of your project.